Blog
Blog
Generative AI and how we can harness its power in clinical development

Between 2016 and 2020, the FDA’s Center for Drug Evaluation and Research (CDER) received only a handful of submissions that included artificial intelligence (AI) or machine learning (ML) — just one submission in 2016, and fourteen in 2020. But in 2021, the number of AI-inclusive submissions swelled to 132 [1].
Of course, as the FDA itself reports, this almost certainly underestimates the true number of applications of AI in the life sciences sector, since the uses extend far beyond the submissions of NDAs and BLAs. And this remarkable leap happened about one year before the emergence of ChatGPT, which is further accelerating the era of generative AI.
This is truly a watershed moment for everyone with an interest in data and technology, many of whom believe that AI has the potential to change the accepted paradigm for drug discovery and development.
Increasing awareness of AI
AI is not a new technology. Its origins probably lie in the 1950s and since then there has been growing interest in AI innovation. Within life sciences, we’ve seen it used quite extensively in target discovery, lead optimization and the development of companion diagnostics.
The emergence of generative AI as an open, foundational technology has lowered the barrier to development of new applications that leverage readily accessible large language models (LLMs) as well as audio- and image-based models. This has caused a reset in thinking about how AI can be applied. It has also unquestionably raised the profile of AI among legislators, regulators and the general public.
What is AI?
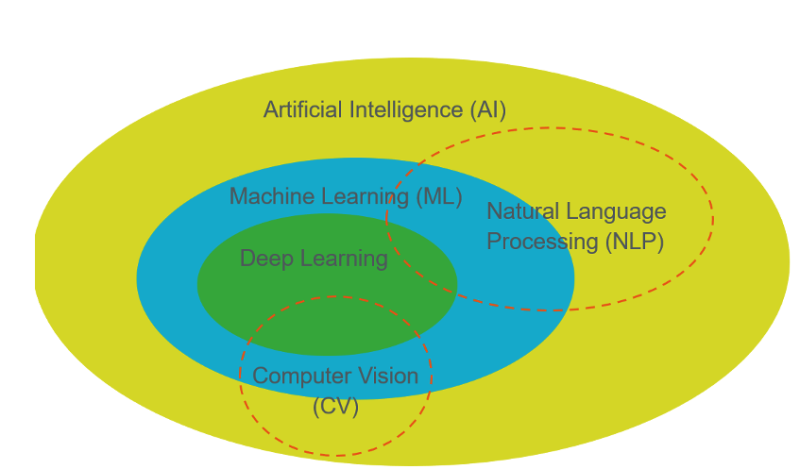
AI includes several different but related elements. As the figure (below) shows, machine learning is a type of AI. Natural language processing (NLP), which is the ability of a computer to interpret text, may rely upon ML. Generative AI, as the name suggests, can process (i.e., interpret) text; but it can also create new text, allowing applications like ChatGPT to reply to user queries with human-like responses. Large language models are generative AI technologies that have been trained on massive amounts of text information, such as the entire publicly visible internet.
AI in clinical development
There’s still much to learn about how to get the best from LLMs, but it’s already evident that they can be especially useful to drug developers in three broad areas.
Search-and-retrieve.
By leveraging proprietary information and data, AI can help businesses quickly provide their staff with the contextualized information they need to perform their roles. For instance, a suitably engineered app can answer questions about business processes by retrieving the details of a standard operating procedure (SOP) document or process map. The ability of the technology to “chat” with users also allows them to ask follow-up questions, which the technology will recognize as part of a continuing dialogue.
Content generation.
LLMs are perhaps best known for this capability and can provide users with good first drafts of original content by transforming existing text. For instance, medical writers may ask an LLM to change the tense of a section from a clinical study protocol, making it more suitable for inclusion in a clinical study report. While this computer-generated text will need review and editing, the opportunities for timesaving and avoidance of cut-and-paste errors are clear. LLMs can also offer style transformation, recreating a document in a new tone or authorial voice to make it more conversational, for example.
Workflow automation.
By embedding LLM technology in workflows, users have the potential to significantly speed up important, high-volume tasks. For example, while it isn’t new to use AI tools for safety and pharmacovigilance (PV) case processing, LLMs can further enhance this process. Likewise, AI can support PV literature reviews and expectedness processing, helping reviewers to better categorize adverse events. With access to the right data, LLMs can also support study feasibility assessment by shortlisting sites and investigators based on experience, capabilities and performance history.
References
[1] Liu et al
Landscape analysis of the application of artificial intelligence and machine learning in regulatory submissions for drug development from 2016 to 2021.
Clinical Pharmacology and Therapeutics: Vol 113 (4); April 2023.








